
Newsroom
Nanog Prevents Early Embryo from Hyperdorsalization
Dorsoventral axis formation is an important event during embryogenesis in vertebrate. Since the dorsal organizer in early embryo was first documented by German scientists Hans Spemann and Hilde Mangold, in the 1920s, many studies have revealed that the dorsal activation of maternal β-catenin induces the formation of dorsal organizer and primary dorsal axis.
However, it remains as a long lasting question that how the activity of maternal β-catenin is controlled in nondorsal cells to prevent the embryo from hyperactivation of maternal β-catenin and hyperdorsalization.
In a study published in PLOS Biology, a research team led by Prof. SUN Yonghua from Institute of Hydrobiology (IHB) of Chinese Academy of Sciences, reported a maternally expressed totipotent factor, Nanog, protects early embryogenesis from global activation of maternal β-catenin activity, therefore ensuring the proper formation of dorsal axis in early embryogenesis.
Using zebrafish as model, researchers revealed a novel mechanism of Nanog safeguarding early embryogenesis through a series of genetic and cellular experiments. In dorsal cells, high amount of nuclear β-catenin competitively binds to Tcf7 even in the presence of nuclear Nanog, inducing the transcription of dorsal organizers and establishing dorsal axis.
In nondorsal cells, high amount of maternally inherited Nanog binds to Tcf7 which safeguards Tcf7 from forming β-catenin/Tcf7 transcriptional activator complex with low amount of nuclear β-catenin. Loss of maternally provided Nanog leads to the derepression of β-catenin/TCF complexes in nondorsal cells, which in turn results in hyperdorsalization.
Nanog precisely regulates the activity of maternal β-catenin signaling in different territories of early embryo, and is essential for proper dorsoventral axis formation. Therefore, this study has preliminarily solved a classical question in the research field of early embryogenesis - the global control of maternal β-catenin activity in early embryo.
The team also mentions that nanog might be a biomarker to evaluate and elevate the quality of fish eggs, since it is significantly downregulated in the eggs of low quality.
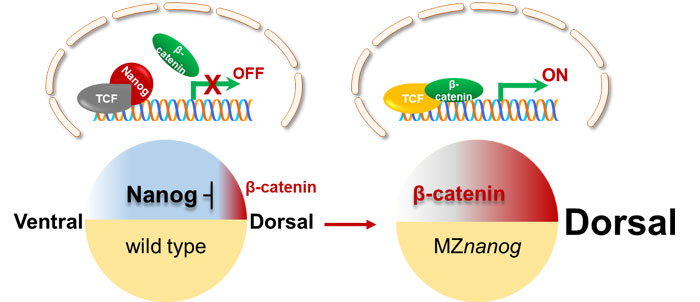
The model of Nanog repressing maternal β-catenin transcriptional activity. In non-dorsal cells of wild type embryo, the amount of nucleus-deposited maternal Nanog (red cartoon object) is much higher than that of the nuclear β-catenin (green cartoon object), therefore Nanog binds to TCF (gray cartoon object) and the β-catenin transcriptional activity is not activated. In MZnanog embryo, however, due to the absence of nanog in the nuclei, small amount of nuclear β-catenin binds to TCF to activate the expression of dorsal organizers, resulting in hyperdorsalization of the embryo. (Figure by IHB)