
Newsroom
Researchers First to Purify and Characterize a Key Enzyme Involved in Microalgae Triacylglycerol Biosynthesis
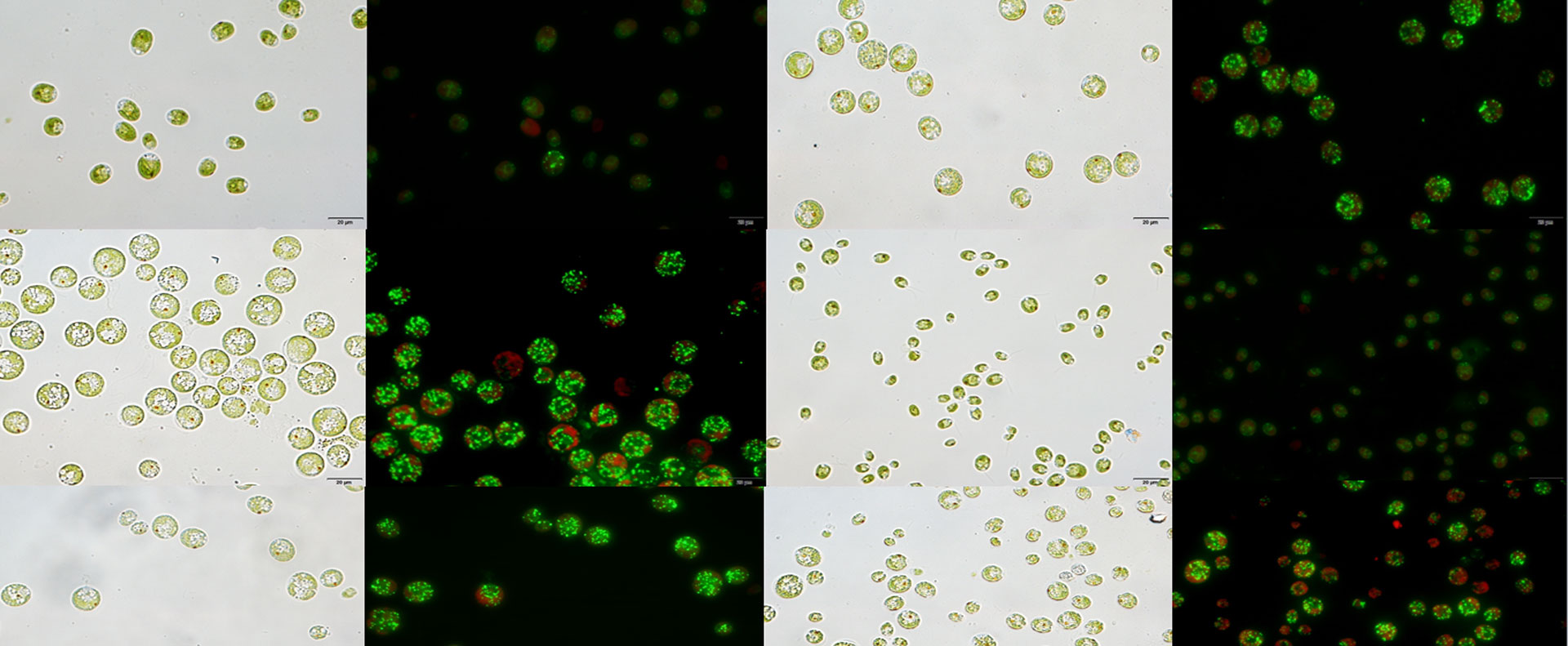
Cell morphology and lipid content of wild type Chlamydomonas reinhardtii and LPAAT1 knockdown mutant under nitrogen deficiency condition (Credit:IHB)
Triacylglycerol, the main energy storage material of photosynthetic unicellular organism microalgae, has been the raw material of microalgae biodiesel. It also has broad application prospects in the field of human health and animal feed.
Recently, researchers from Institute of Hydrobiology (IHB) of Chinese Academy of Sciences purified a key enzyme involved in triacylglycerol biosynthesis of the unicellular green alga Chlamydomonas reinhardtii, the plastidial lysophosphatidic acid acyltransferase (CrLPAAT1) which is a membrane-bound protein, in a soluble form and utilized it for comprehensive biochemical characterization.
The research results, published in Algal Research, showed that the recombinant CrLPAAT1 prefers to utilize C16:0-CoA over other acyl donors. Furthermore, the two transmembrane domains of CrLPAAT1 are involved in shaping its substrate preference for C16:0-CoA.
Additionally, CrLPAAT1 was found to be interacting with the water-soluble plastidial glycerol-3-phosphate acyltransferase (CrGAPTcl) via its two transmembrane domains in vitro.
The interaction between CrLPAAT1 and CrGPATcl can be negatively regulated by both the acyl-CoAs and lysophosphatidic acid in a dosage-dependent manner. Such a regulation pattern may represent a novel mechanism adopted by algal cells to control lipid metabolism homeostasis under various environmental conditions (Figure 1).
Understanding the synthesis mechanism of the microalgae triacylglycerol at the molecular level is of great significance for improving oil production by biotechnology.
This finding provides a new way to study the regulation mechanism of lipid metabolism in microalgae.
