
Newsroom
Heterocyst-development Regulator HetF Functions as New Divisome Component
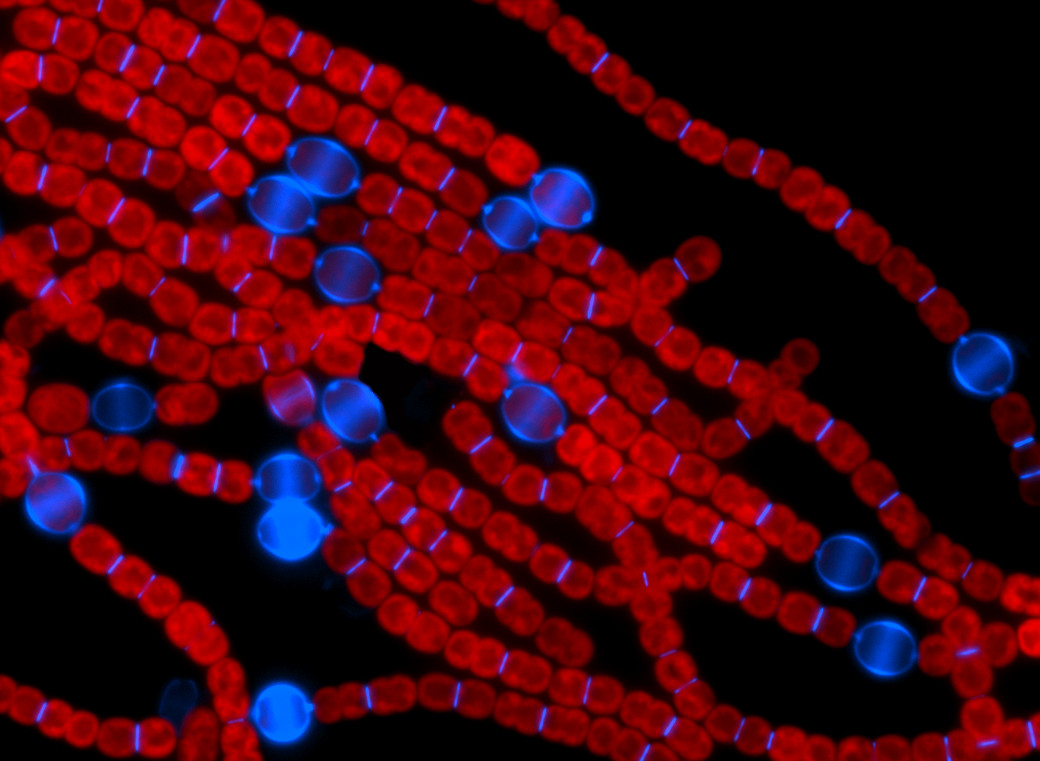
Fluorescent image of the filamentous cyanobacterium Anabaena PCC 7120. (Credit: IHB)
Bacterial cell division, with a few exceptions, is driven by FtsZ through a treadmilling mechanism to remodel and constrict the rigid peptidoglycan (PG) layer through the cell division complex, divisome. After nitrogen step-down, the filamentous cyanobacterium Anabaena PCC 7120 (Anabaena) is able to form heterocysts, cells that are capable of N2-fixing but have lost the capacity of division. Previous studies already showed that heterocyst differentiation depends on the process of cell division, but the genetic mechanism is still unclear.
Recently, the research group led by Professor ZHANG Cheng-Cai from the Institute of Hydrobiology (IHB) of the Chinese Academy of Sciences reported that the heterocyst-development regulator HetF is a new divisome component. The results were published in mBio.
In Anabaena, hetF is required for the initiation of the differentiation of heterocysts. It was shown in this study that expression of hetF is active in vegetative cells and down-regulated in heterocysts, suggesting that HetF functions in vegetative cells.
The researchers knocked out hetF in Anabaena using CRISPR/Cpf1 and found that HetF is required for cell division under certain conditions. Particularly, under the high-light condition, cells of a ΔhetF mutant stopped dividing which was consistent with increased level of HetF under similar conditions in the wild type.
Moreover, the researchers checked the localization of HetF that fused with green fluorescent protein (GFP) in vivo and found that HetF is a membrane protein enriched at midcell and cell-cell junctions.
In the absence of HetF, the rings of FtsZ are still present in the elongated cells; however, PG remodeling activity at the septa, shown by 7-hydroxycoumarin-amino-D-alanine (HADA) labeling, is abolished. This phenotype is similar to that was observed with the inhibition of septal PG synthase FtsI. Protein-protein interaction assay showed that HetF actually interact with FtsI directly.
By screening HetF point mutations that do not interact with FtsI, the researchers further revealed that HetF is recruited to the divisome by interacting with FtsI and their interaction is necessary for HetF functioning in cell division.