
Newsroom
To Divide and To Differentiate: A Coordination Mechanism Identified From A Multicellular Cyanobacterium
Bacterial cell division, with a few exceptions, is driven by FtsZ through a treadmilling mechanism to remodel and constrict the rigid peptidoglycan (PG) layer through the cell division complex, divisome. Upon combined-nitrogen deprivation, the filamentous and multicellular cyanobacterium Anabaena sp. PCC 7120 forms heterocysts, a particular type of cells capable of N2-fixing but incapable of division. Despite the opposing properties of reproductive vegetative cells and terminally differentiated heterocysts, heterocyst differentiation is coupled to cell division but the underlying mechanism remains unclear.
Recently, the research group led by Professor ZHANG Cheng-Cai from the Institute of Hydrobiology (IHB) of the Chinese Academy of Sciences reported in the journal PNAS that HetF, as a protease, regulated cell division and heterocyst differentiation by controlling the inhibitory effects of PatU3.
HetF is a long-known heterocyst-development regulator, and it was shown to function as a divisome component in Anabaena by ZHANG’s group last year. In the new publication, using suppressor screening, the researchers identified PatU3, a negative regulator for heterocyst differentiation first reported byProfessor XU Xudong’s lab from IHB, acting downstream of HetF.
The inactivation of patU3 restored the capacity of cell division and heterocyst differentiation in the hetF mutant; and ectopic expression of patU3 in wild-type cells mimics hetF deletion: the rings of FtsZ are still present in the elongated cells; however, PG remodeling activity at the septa is abolished as shown by PG labeling with 7-hydroxycoumarin-amino-D-alanine (HADA), an analog of the peptidoglycan precursor D-Ala.
Furthermore, the researchers demonstrated that PatU3 was a specific substrate of the protease activity of HetF both in vitro and in vivo, and mapped the cleavage site in PatU3 by N-terminal sequencing of the cleavage product.
Taken together, the study reveals that the protease HetF regulates cell division and heterocyst differentiation by controlling the inhibitory effects of PatU3 (Fig. 1). Such a proteolytic pathway constituted a mechanism for the coordination between cell division and differentiation in a prokaryotic model used for studies on developmental biology and multicellularity.
Cyanobacteria are not only important players for element cycles in different environments, they are also increasingly used for biotechnological applications. Some cyanobacteria can proliferate massively in eutrophic water bodies, casing blooms with toxin production, a major concern for water management. A better understanding of fundamental processes of cyanobacterial cells will provide handles to broaden cyanobacterial biotechnology and to develop strategies for bloom control.
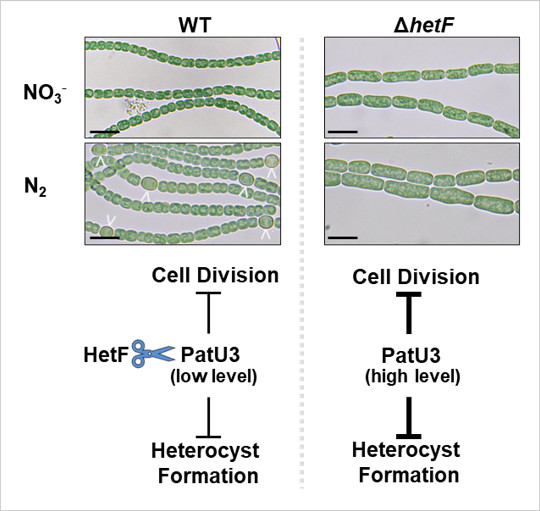
HetF regulates cell division and heterocyst differentiation by controling the levels of PatU3 through proteolysis. In wild-type (WT), PatU3, the inhibitor of cell division and heterocyst formation, is cleaved by the protease HetF (indicated by scissors) to maintain a lower level so that cell division and heterocyst formation occur properly. In the hetF-deficient strain (ΔhetF), PatU3 accumulates to a level high enough to inhibit cell division and heterocyst development. Anabaena strains were grown in the media with (NO3-) or without (N2) combined nitrogen, respectively. Arrows indicate heterocysts. Scale bar is 10 μm. (Figure by IHB)
(Editor: MA Yun)