
Newsroom
Exploring Immunomodulatory and Antioxidant Potential of Gallic Acid in Fish Gut-Liver Axis
Aquatic products constitute the third-largest source of dietary protein for humans, with global aquaculture production, especially in China, experiencing rapid growth over the past two decades. However, this expansion has been accompanied by challenges such as deteriorating water quality and increased input costs due to the implementation of antimicrobial resistance restrictions, which have become significant bottlenecks in the sustainable development of aquaculture. Consequently, there is a pressing need for research into functional plants with anti-aquatic properties.
One of the primary concerns in aquatic products is foodborne enteroheptal lesions in fish, which can lead to reduced disease resistance and stunted growth. To address this, researchers from the Institute of Hydrobiology(IHB)of the Chinese Academy of Sciences, have found that gallic acid, as an agonist of PPARγ in fish, can regulate the enteroheptal immune homeostasis by binding to zebrafish PPARγ protein. The study was published in Aquaculture.
In this study, the researchers have investigated the effects of gallic acid, a core ingredient of sea buckthorn, in soybean meal-induced enteritis (SBMIE) of zebrafish foodborne enteritis models. Their research involved pathological assessment, analysis of immune gene expression, immune cell imaging, flow cytometry, and multi-omics analysis (including transcriptomics, microbiomics, and metabolomics).
The researchers performed histological analysis and revealed that the intestinal mucosal villi length and Cluster of Differentiation 4 (CD4) signal exhibited patterns akin to those observed in healthy control subjects. Moreover, inflammatory infiltration of neutrophils, macrophages, and T cells in the hindgut was effectively suppressed. In 3-month-old zebrafish, well-established markers of liver inflammation associated with soybean meal-induced inflammation, such as reduced lipid droplets and macrophage accumulation were ameliorated.
Based on transcriptome data analysis, the researchers uncovered significant downregulation of cytokines, apoptosis-related genes, and oxidative phosphorylation pathways within the intestinal tissue. Conversely, pathways related to short-chain fatty acid metabolism and arginine, which play pivotal roles in immune regulation were notably upregulated. Concurrently in liver, gallic acid administration resulted in the inhibition of fatty acid metabolism, primarily regulated by PPARγ signaling pathways while macrophage aggregation was also attenuated.
At the same time, analysis of intestinal microbiota sequencing data revealed a reduced presence of pathogens in the gallic acid-treated group. Relative proportions of Firmicutes and Bacteroides resembled those of healthy controls. Furthermore, the heightened presence of warty microbacteria may contribute to intestinal barrier protection with an observed increase in commensal bacterium Shewanella.
Further, intestinal metabolome analysis showed an increase in anti-inflammatory and antioxidant compounds, and analysis of differentially expressed genes with altered intestinal metabolites revealed potential anti-enteritic metabolites and related immunomodulatory genes including il21, il22, tnfrsf118 and mapkapk3.. This complex interaction sheds light on the molecular mechanism by which metabolites in intestinal tissues are involved in anti-inflammatory interactions between fish and microorganisms.
This study has revealed that gallic acid primarily functions by promoting immune regulation and enhancing antioxidant activity in the context of mitigating intestinal and hepatic inflammation in fish, and gallic acid reduces foodborne inflammation in the enterohepatic axis of fish by stimulating PPARγ-mediated immunomodulatory and antioxidant processes.
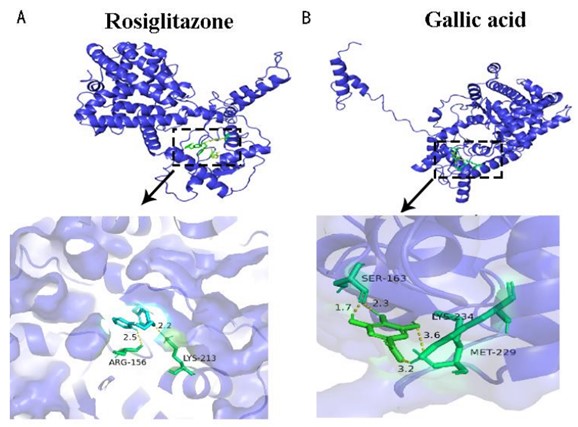
Molecular docking results of gallic acid and zebrafish PPARγ protein (Image by IHB)
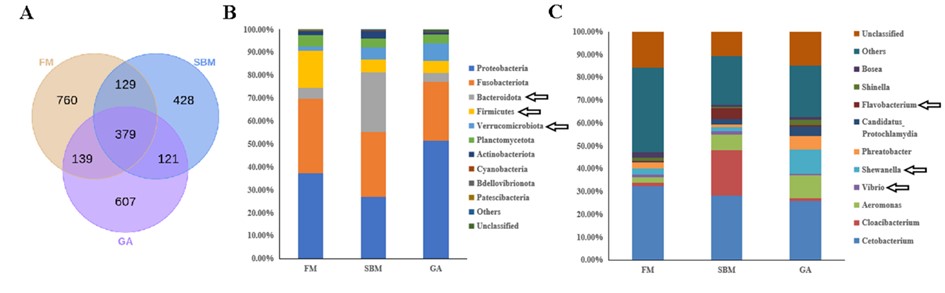
Changes in Zebrafish SBMIE Adult Model's Intestinal Microbiota Composition with Gallic Acid Dietary Incorporation (Image by IHB)
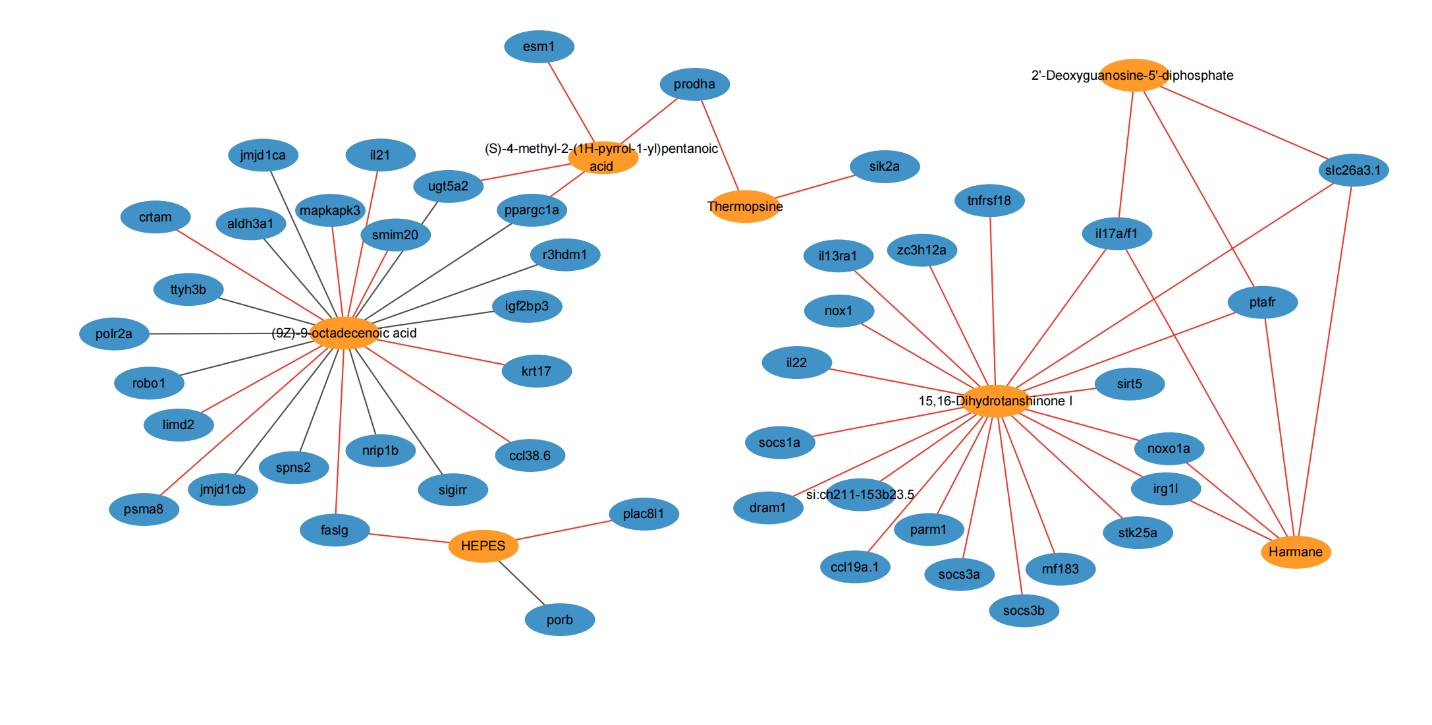
Association analysis between differential metabolites and differential immune genes in intestinal tissue (Image by IHB)
(Editor: MA Yun)