
Newsroom
A Mettl16/m6A/mybl2b/Igf2bp1 Axis Ensures Cell Cycle Progression of Embryonic Hematopoietic Stem and Progenitor Cells
Hematopoietic stem and progenitor cells (HSPCs) have self-renewal capacity and multilineage differentiation potential that supports the lifelong supply of all types of blood cells. Cell cycle dysregulation of HSPCs is frequently associated with hematopoiesis-related diseases, such as bone marrow failure syndrome (BMFS), myelodysplastic syndrome (MDS) and various hematologic malignancies. Thus, it is critical to reveal the regulatory mechanisms underlying the cell cycle progression of HSPCs.
N6-methyladenosine (m6A) is the most abundant internal mRNA modification in eukaryotes. Emerging evidence indicates that m6A modification plays an importance role in the expansion and differentiation of HSPCs in adulthood. Nevertheless, the role of m6A in the development of embryonic HSPCs remains elusive. METTL16 is a recently identified m6A methyltransferase (also known as the m6A writer), and its knockout results in prenatal lethality in mice. The physiological function of METTL16 in early embryonic development is still unknown.
Recently, research groups led by Prof. Mugen Liu from Huazhong University of Science and Technology and Prof. Daji Luo from the Institute of Hydrobiology (IHB) of the Chinese Academy of Sciences illustrated the novel molecular mechanism linking the METTL16-mediated m6A modification to the cell cycle progression of HSPCs during early embryonic development. This study was published on The EMBO Journal.
In this study, the authors found that METTL16 is highly expressed in HSPCs among vertebrates through cross-species analysis of three independent scRNA-seq datasets of humans, mice, and zebrafish at early embryonic stage. A mettl16 knockout zebrafish line was generated by CRISPR/Cas9 technology and the developmental process of HSPCs was tracked. They found that the maintenance of HSPCs in the CHT, but not their emergence or settlement, was impaired in the mettl16 mutants. Further experiments showed that reduced proliferation of HSPCs due to G1/S cell cycle arrest is responsible for the developmental defect of HSPCs in the mettl16 deficient embryos.
To test whether the developmental defect of HSPCs is m6A-dependent or m6A-independent, the authors performed rescue experiments using the wild-type and enzyme-inactivated versions of Mettl16, respectively. They found that the regulatory effect of Mettl16 on HSPC development mainly relies on its methyltransferase activity. Furthermore, they performed MeRIP-seq to identify the changes of m6A modification on RNA molecules between WT and mettl16 knockout zebrafish embryos at 3 dpf. The mybl2b gene, encoding a MYB family transcription factor involved in cell cycle progression, was shown to be a novel target of Mettl16 in HSPCs. Further RIP-qPCR experiments demonstrated that Mettl16 could directly bind mybl2b mRNA. In addition, they identified Igf2bp1 as a potential m6A reader of mybl2b, which mediates the effect of Mettl16 on mybl2b mRNA stability via m6A modification.
Finally, the authors tested whether the METTL16-m6A-MYBL2 signaling axis in G1/S progression is conserved in humans. They found that METTL16 and IGF2BP1 could directly bind MYBL2 mRNA in K562 cells, and knockdown of METTL16 reduced the m6A modification level and mRNA level of MYBL2, and triggered G1/S arrest. To further verify this mechanism in the cell cycle progression of human HSPCs, the authors isolated CD34+ cells from human cord blood and knocked down METTL16 in the CD34+ HSPCs. Similar to the situations in zebrafish and K562 cells, they observed the G0/G1 phase arrest and decreased m6A modification and mRNA levels of MYBL2.
In summary, their work, for the first time, revealed a critical role of Mettl16 in HSPC proliferation in zebrafish and established a m6A regulatory network of Mettl16 on HSPC cell cycle progression. The METTL16-m6A-MYBL2 signaling axis in G1/S progression is demonstrated to be also conserved in humans. These findings may lead to new strategies of therapeutic interventions for hematopoiesis disorders.
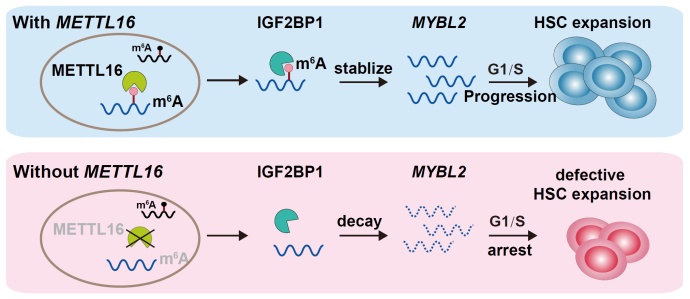
The molecular mechanism of Mettl16 regulating HSPC cell cycle progression (Image by IHB)
(Editor: WANG Hongxia)