
Newsroom
Manipulating the Unisexual – Sexual Reproduction Transition to Engineer Novel Polyploids with Enhanced Resistance and High Yield
Sexual reproduction predominates among eukaryotic organisms. During meiosis and fertilization, recombination and gamete fusion give rise to genetically variable progenies, which serve as the basis for selection in agriculture. However, the beneficial traits obtained through sexual reproduction, especially heterosis, would segregate in subsequent generations. Harnessing unisexual reproduction has been hailed as the holy grail of agriculture, as it is expected to revolutionize biotechnology by allowing the perpetuation of any favored genotypes or genomes, especially in heterosis fixing.
Recently, the coupling of unisexuality and polyploidy has been demonstrated to enable the design of polyploid genomes for heterosis exploitation and fixation in crops. However, utilizing unisexual reproduction to engineer polyploid genomes remains challenging in animals.
In a study published in Advanced Science, a research team led by Prof. GUI Jianfang from the Institute of Hydrobiology (IHB) of the Chinese Academy of Sciences provided the first successful case of engineering polyploid genomes with desirable traits via unisexual–sexual reproduction transition in animals, offering key breeding technologies for cultivating the novel strain (gibel carp “CAS VI”).
In this study, the researchers crossed a sexual amphitetraploid male (AAAABBBB) with a sexual amphidiploid C. cuvieri (AABB) that possesses natural resistance to C. auratus herpesvirus (CaHV). This mating produced a group of novel polyploids containing genomic components from three species.
Assembly and analysis of haplotype-resolved genome revealed the novel amphitriploid inherited one genome set (AB) from the maternalC. cuvieri and two genome sets (AABB) from the paternal amphitetraploid. In the latter compositions, one or two recombination events had taken place in some homologous chromosomes between C. auratus and C. gibelio. Therefore, the novel amphitriploid was termed as thegenome-reconstructed amphitriploid (GR-A3n) (AAABBB).
Then, the researchers found that the gynogenesis ability have transferred from C. gibelio to some of the GR-A3n females. This study subsequently established three GR-A3n clones with distinct herpesvirus resistance.
Differentialtranscriptome profiles were subsequently characterized in two main hematopoietic organs of the three GR-A3n clones.Most genesofthe hemoglobin metabolism pathway were found to exhibit high expression levels, as in C. cuvieri, which led to efficient hemoglobin biosynthesis and blood oxygen homeostasis during infection, thereby resulting in strong herpesvirus resistance.
Furthermore, this study determined the resistant and susceptible haplotypes derived from chromosome 12BofC. cuvieri, which should be responsible for resistance differences between GR-A3n clones. Therefore, through the multi-genome reconstruction and reproduction transitions driven by ploidy changes, both the gynogenesis ability ofC. gibelio and the herpesvirus resistance of a wild sexual C. cuvieri were introduced into the genome-reconstructed amphitriploid.
The favored resistant haplotype, coupled with a growth advantage, was fixed into the GR-A3n clone-2, which has been developed as a new variety for aquaculture owing to its enhanced resistance and increased yield. This study demonstrates that unisexual–sexual reproduction transition enables polyploid genome design in animals, potentially paving the way for completely novel breeding schemes.
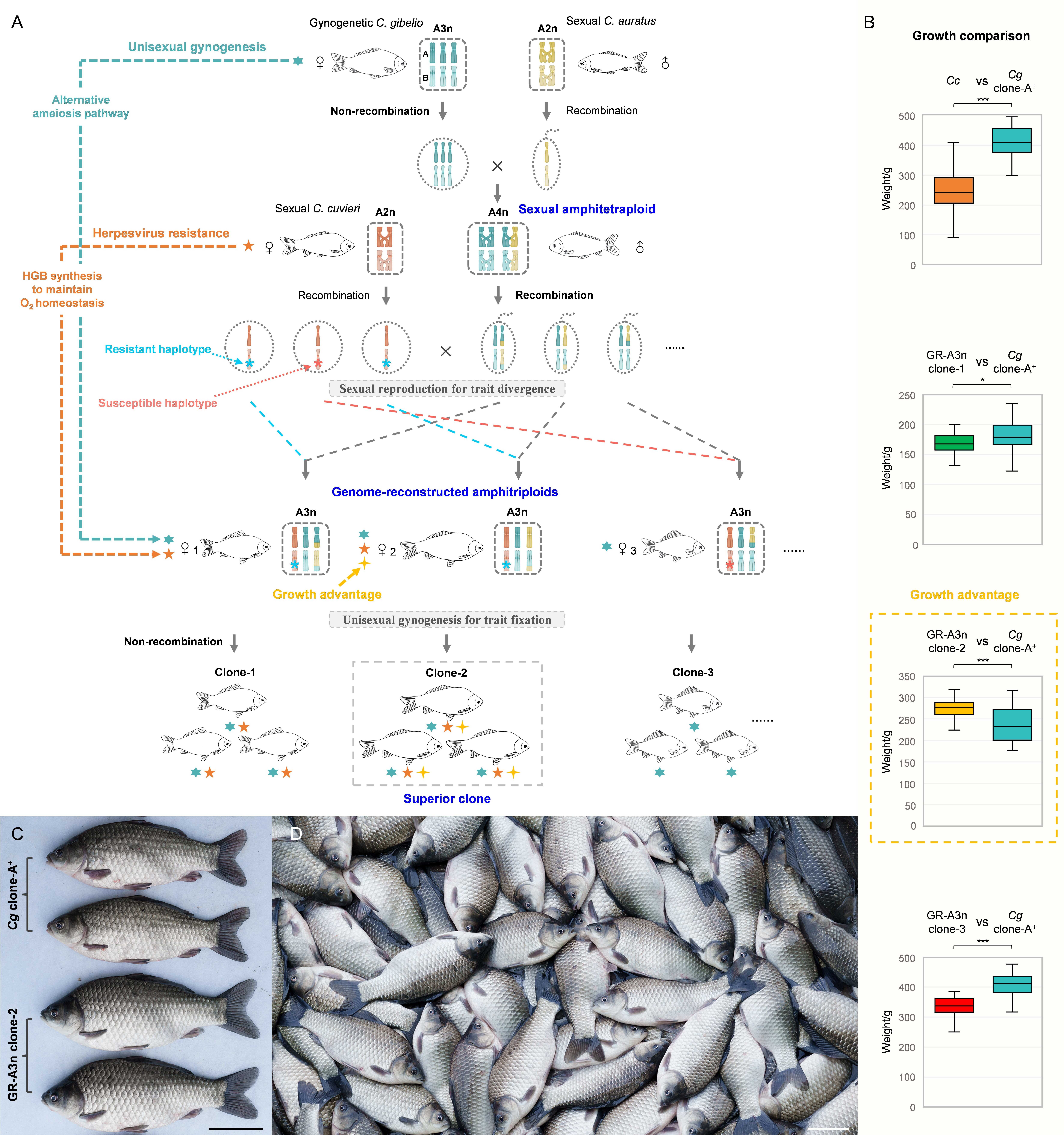
Manipulating unisexual–sexual reproduction transition to engineer genome-reconstructed polyploids with elite traits (Image by IHB)
(Editor: MA Yun)