Highlights
Zebrafish hif-3α Facilities Hypoxia Tolerance by Modulating Erythropoiesis via gata-1 Regulation
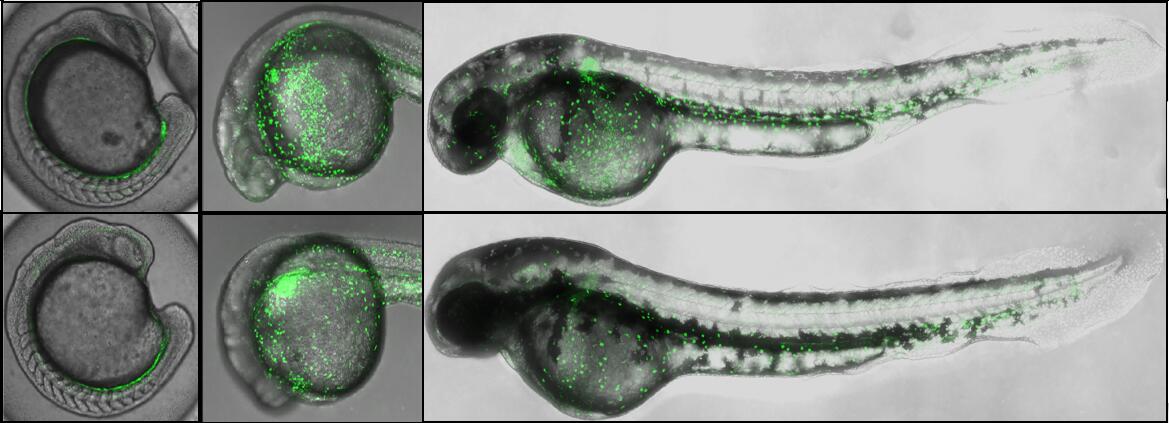
Fluorescent images of Tg (gata1:eGFP) / hif3a+/+ (top) and Tg (gata1:eGFP) / hif3a-/- (bottom) indicated that hif3a-/- have fewer gata1-positive erythrocytes at 24 hpf and 48 hpf. (Credit: IHB)
The hypoxia-inducible factors 1α and 2α (HIF-1α and HIF-2α) are master regulators of the cellular response to O2. To date, their function in hypoxia signaling is relatively clear. HIF-3α, another hypoxia-inducible factor, has some different isoforms. The molecular mechanism and biological function of HIF-3α in hypoxia signaling pathway is largely unknown.
The research group led by Prof. XIAO Wuhan from Institute of Hydrobiology (IHB) of Chinese Academy of Sciences, revealed that HIF-3α facilities hypoxia tolerance by modulating erythropoiesis via gata-1 regulation. The results were published online in Development.
Using CRISPR/Cas9 technology, the researchers knocked out hif-3α in zebrafish. In the following experiments, they found that more hif-3α-/- larvae were dead than hif-3α+/+ larvae after exposing larvae to 2% O2 for 12 hours. These data suggested that disruption of hif-3α attenuated hypoxia tolerance in zebrafish.
When they routinely examined the hif-3α+/+ and hif-3α-/- larvae under a dissection microscope, they noticed that the hif-3α-/- larvae always had fewer blood cells compared to hif-3α+/+ larvae. Using O-dianisidine staining to measure the red blood cells, they found fewer O-dianisidine-positive cells in the hif-3α-/- larvae than in the hif-3α+/+ larvae, indicating that knockout of hif-3α disrupted erythropoiesis in zebrafish.
To determine whether the defective erythropoiesis displayed by the hif-3α-/- was associated with erythroid maturation, the researchers analyzed the morphology of isolated red blood cells by using May-Grunwald-Giemsa staining. They found that hif-3α-/- had a higher percentage of proerythroblasts and a lower percentage of mature erythroid precursors than the WT, suggesting that the deletion of hif-3α might impede erythroid cell maturation.
In addition, they used the whole mount in situ hybridization to examine the expression of hematopoietic markers (including scl, lmo2, runx1, c-myb, epo, gata1, alas2, band3, hbae1, hbbe1, hbae3, hbbe3), and figured out the mechanisms of hif-3α on erythropoiesis - the disruption of zebrafish hif-3α abrogated the expression of hematopoietic marker genes, resulting in defects of erythropoiesis; and gata-1 might be the downstream effector mediating the function of hif-3α in erythropoiesis.
Hypoxia-response element (HRE), a response element recognized by hypoxia-inducible factors, was located in the promoter of downstream targets of hypoxia-indecible factors. The researchers revealed that there is a potential HRE located at -105— -101 in the promoter of gata1, and confirmed that zebrafish hif-3α directly activated gata1 expression by recognizing the HRE site, thus modulating the erythropoiesis.
This study not only demonstrated that zebrafish hif-3α facilitate hypoxia tolerance via regulation of gata1, but also suggested that hif-3α might behave similar to hif-1α and hif-2α in hypoxia signaling pathway.