Highlights
Bis (2-ethylhexyl)-2,3,4,5-tetrabromophthalate Show Poor Penetrability but Increase Permeability of Blood Brain Barrier
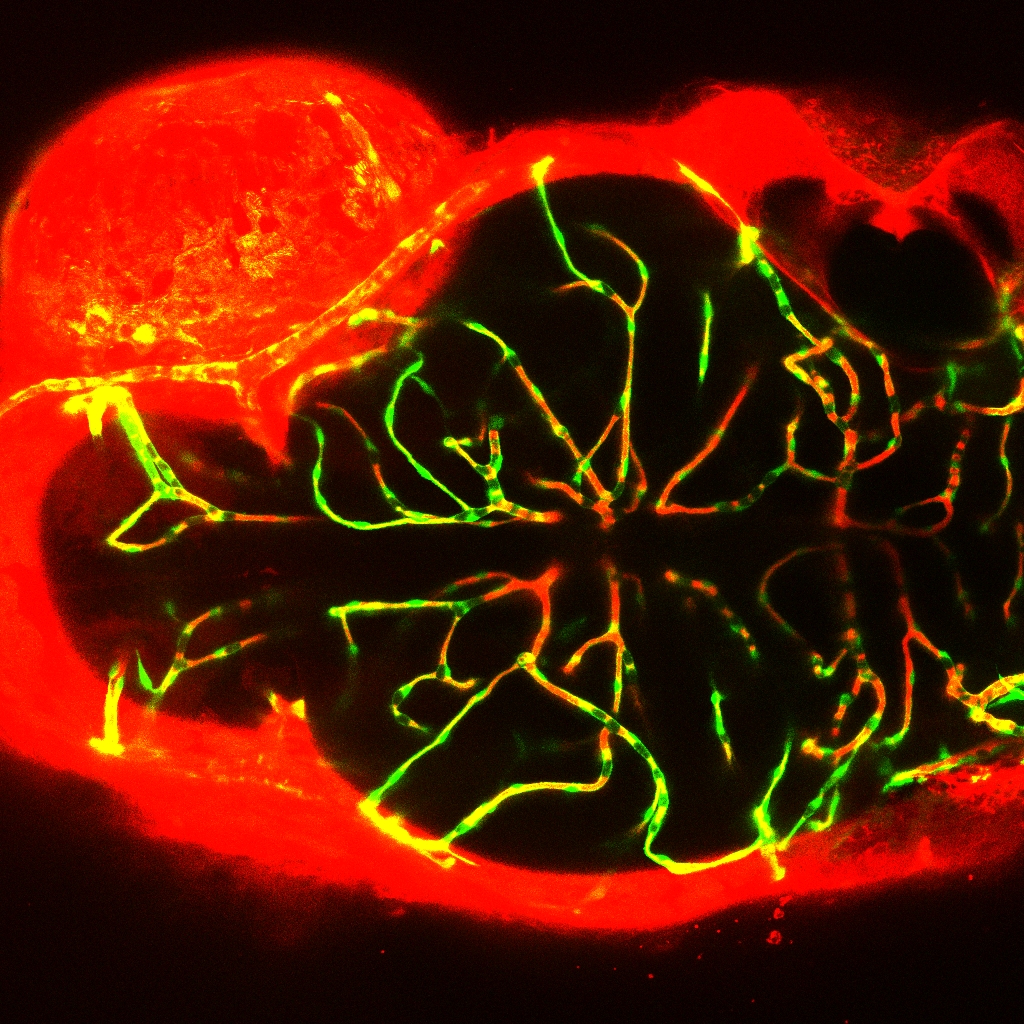
Embryonic exposure to bis (2-ethylhexyl)-2,3,4,5-tetrabromophthalate increased the permeability of blood-brain barrier in transgenic zebrafish (Tg flk1: EGFP) (Credit: IHB)
The blood-brain barrier (BBB), which is composed of monolayer endothelial cells between the plasma and brain cells, is crucial for maintaining the homeostasis of the central nervous system (CNS). The novel brominated flame retardants (NBFRs) are believed to cause damage to the CNS via crossing the BBB because of their high fat-solubility. Although NBFRs have been detected in brain, there lacked evidence for their penetrability through the BBB, making it hard to evaluate and understand their direct effects on CNS.
The research group led by Prof. ZHOU Bingsheng from the Institute of Hydrobiology (IHB) of the Chinese Academy of Sciences found that bis (2-ethylhexyl)-2,3,4,5-tetrabromophthalate (TBPH) had poor penetrability through BBB, but could increase the permeability of BBB by affecting the tight junctions. This may result in disturbed homeostasis across BBB, thus causing adverse effects on the nervous system. The study was published in Journal of Hazardous Materials.
In this study, the researchers first used an in vitro model of human immortalized endothelial cells, hCMEC/D3, to evaluate the penetrability of TBPH. In comparison with Fluorescein sodium, TBPH was found to have a lower Papp during different periods, suggesting that TBPH had poor penetrability through BBB.
Using transgenic zebrafish (Tg flk1: EGFP) as an in vivo model, the researchers confirmed that TBPH could affect the BBB permeability probably via affecting the transcription of genes encoding tight junction proteins.
Using wild type zebrafish embryos/larvae, they assessed the potential neurotoxicity of TBPH. The results showed that embryonic exposure to TBPH did not remarkably change the hatching, survival and malformation rates, neurotransmitter contents, or locomotor activity, possibly because TBPH can hardly cross the BBB to pose direct exposure to the CNS.
However, the transcription of opsins genes and visual response to light stimulation in zebrafish larvae were inhibited. The alteration of phototactic response may have adverse effects on predator avoidance response which could increase the environmental risk of fish. The underlying mechanism should be further explored.