Highlights
PDAT Regulates PE as Transient Carbon Sink Alternative to Triacylglycerol in Nannochloropsis
Nannochloropsis is a group of unicellular eukaryotes that belong to Eustigmatophyceae. Currently, there are seven identified species in this genus which have high photosynthetic efficiency, biomass and oil content (triacylglycerol, TAG), and are rich in eicosapentaenoic acid (EPA), making them high-quality raw materials for the industrial production of EPA.
N. oceanica has been recognized as an industrially important single-cell factory for lipid production because of its fast growth and high lipid content. Its genome contains up to 13 acyl CoA:diacylglycerol (DAG) acyltransferases (NoDGATs), which contribute to stress and non-stress associated TAG biosynthesis, and raises questions about whether the unique N. oceanica phospholipid:DAG acyltransferase (NoPDAT) is critical, and under what conditions and to what extent it contributes to TAG biosynthesis.
A research team led by Prof. HU Hanhua from the Institute of hydrobiology (IHB) of the Chinese Academy of Sciences recently addressed the function and physiological role of NoPDAT in N. oceanica. The study was published in Plant Physiology.
Prof. Hu’s team has carried out a series of basic researches on the above-mentioned algal strains for more than ten years. They first established an efficient genetic transformation system based on PCR products (Bioscience, Biotechnology, and Biochemistry, 2014) and a gene knockdown (KD) system based on RNA interference (Plant Journal, 2017) in all the six marine species.
The researchers achieved the high-efficiency genetic transformation based on electroporation by chemical pretreatment in N. limnetica, the only freshwater alga in this genus (World Journal of Microbiology and Biotechnology, 2019).
They found that NoPDAT resides at the outermost plastid membrane, chloroplast endoplasmic reticulum (cER). Based on Genetic analysis, NoPDAT contributed at least 30% to TAG biosynthesis under nitrogen limited conditions, and NoPDAT KD has not triggered any compensatory mechanism via DGATs.

Cell localization of NoPDAT (A, green and red fluorescence are NoPDAT-eGFP fusion protein and chloroplast signal, respectively), reduction of neutral lipids (B) and changes in total fatty acid (TFA) and TAG content (C) caused by NoPDAT KD) (Figure by IHB)
Through the semi-quantitative TLC analysis of polar lipids, the researchers also found that NoPDAT KD leads to a vast accumulation of a new class of phosphatidylethanolamines (PEs) in cells, and this special PE containing 16:0, 16:1, and 18:1 fatty acids (referred to as “LU-PE”) differs from the intracellular functional PEs containing polyunsaturated fatty acids (C20:4 and C20:5).
Moreover, the content of intracellular LU-PE was significantly positively correlated with the CO2 concentration in culture.
Results of overexpressing and/or KD of genes involved in PE homeostasis indicated that LU-PE accumulation in N. oceanica is not linked to these genes and that NoPDAT is solely responsible for the observed profile change.
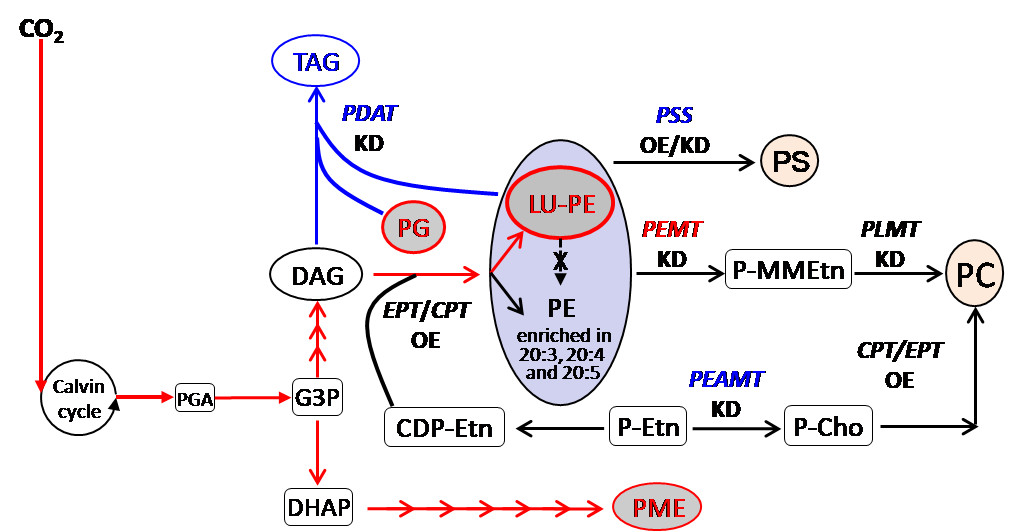
A hypothesized working model illustrating the role of NoPDAT in lipid metabolism in N. oceanica. (Figure by IHB)
In summary, this study uncovers that the NoPDAT pathway is parallel to and independent of the NoDGAT pathway for oil production. LU-PE can serve as an alternative carbon sink for photosynthetically assimilated carbon in N. oceanica when PDAT-mediated TAG biosynthesis is compromised or under stress in the presence of high CO2 levels.
(Editor: MA Yun)