Highlights
Deubiquitinase OTUD3 Exhibits Opposing Effect in Innate Immunity Against RNA and DNA Viruses
Initiating an immune response to viral infection begins with the detection of pathogen-associated molecular patterns (PAMPs) by pattern-recognition receptors (PRRs). RIG-I, MDA5 and cGAS are major PRRs that can sense viral nucleic acids. As their activation is strictly regulated by polyubiquitination and deubiquitination, it is important to understand how RIG-I, MDA5 and cGAS are regulated by ubiquitinase and deubiquitinase.
A research group led by Prof. XIAO Wuhan from the Institute of Hydrobiology (IHB) of the Chinese Academy of Sciences recently found that the deubiquitinase OTUD3 exhibits an opposing effect in the innate antiviral response to RNA and DNA viruses. The study was published in Cell Reports.
In this study, the researchers first used ubiquitination assays and found that overexpression of OTUD3 diminished K63-linked polyubiquitination of RIG-I and MDA5 simultaneously. “It indicated that OTUD3 is a negative regulator in innate immunity medicated by RIG-I and MDA5,” said Prof. Xiao.
Unanchored K63 ubiquitin chains induce oligomerization of RIG-I and MDA5, leading to their activation during an antiviral innate immune response. The researchers then examined whether OTUD3 affects the oligomerization of RIG-I and MDA5, and their results suggested that OTUD3 inhibits the oligomerization and prevents viral RNA binding to RIG-I and MDA5.
The E3 ligases RNF135 and TRIM65 have been suggested to target RIG-I and MDA5 for K63-linked polyubiquitination respectively. Researchers then transfected RNF135 together with RIG-I, or TRIM65 together with MDA5 in 293T cells and performed ubiquitination assays. They also confirmed that OTUD3 can inhibits RNF135-induced RIG-I and TRIM65-induced MDA5 K63-linked polyubiquitination.
To evaluate the importance of Otud3 in the host against RNA virus infections in vivo, the researchers used mice and zebrafish as model animal, and demonstrated that OTUD3-deficient mice and zebrafish are more resistant to RNA viruses infection.
The researchers subsequently detected the effect of OTUD3 in DNA virus-triggered innate antiviral signaling. “What we found interesting is that OTUD3 is a positive regulator in response to DNA viruses infection, and OTUD3 can interact with cGAS and catalyzed the removal of K48-linked polyubiquitin chains,” said Prof. Xiao.
To further clarify the mechanism of OTUD3 in DNA virus-triggered innate antiviral signaling, the researchers performed mutation and ubiquitination assays, which showed that OTUD3 cleaved K48-linked polyubiquitination chains from Lys279 of cGAS, and then promoted the DNA-binding ability of cGAS and protected cGAS from degradation.
In addition, mice and zebrafish were also used to confirm that OTUD3 positively regulated DNA virus-trigged antiviral immune responses in vivo.
This study revealed that OTUD3 plays an opposing role in innate antiviral immunity against RNA and DNA viruses, which highlights the complexity of the host antiviral response. “OTUD3 can be a selected molecule for developing therapeutic treatments for viral infections,” Prof. Xiao concluded.
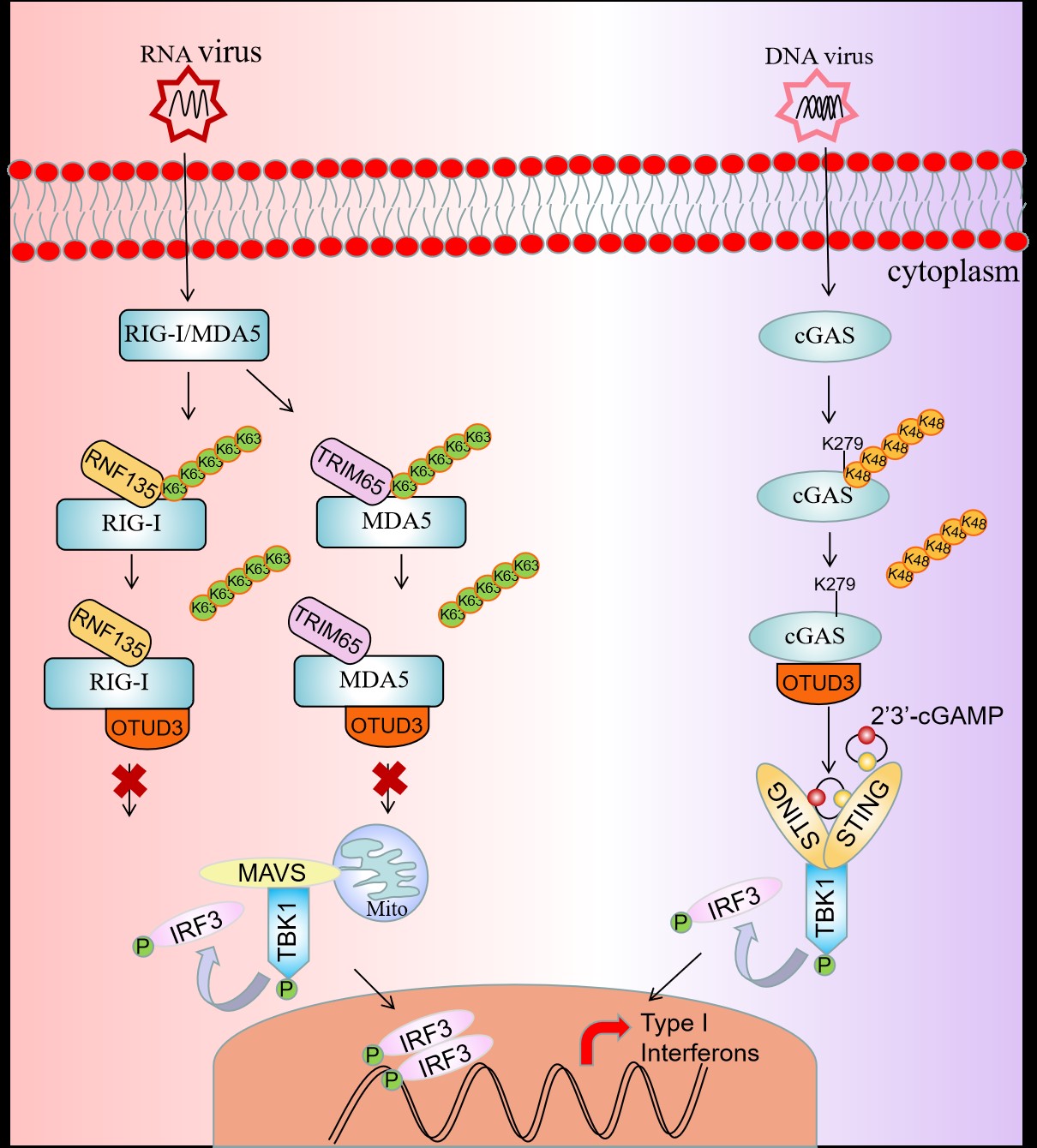
Graphical abstract of deubiquitinase OTUD3 in antiviral innate immunity (Image by IHB)
(Editor: MA Yun)