Highlights
Zebrafish LGP2 Acts as Homeostatic Regulator of Innate Interferon Response During Viral Infection
In 2003, cytosolic retinoic acid inducible gene-I (RIG-I)-like receptors (RLRs) were identified as viral RNA sensors, opening a window for the study of how vertebrates rapidly initiate interferon (IFN)-mediated antiviral immune responses. RLR family is composed of RIG-I, melanoma differentiation-associated gene 5 (MDA5) and laboratory of genetics and physiology 2 (LGP2). In mammals, it is RIG-I and MDA5 that sense different viruses to trigger IFN antiviral immune response, thus defensing against virus infection. How LGP2 functions toward virus infection remains controversial.
The current viewpoint is that, when infected with virus, LGP2 not only facilitates MDA5 to recognize viral infection, but acts as an inhibitor of RIG-I-mediated antiviral immune response as well. Further in vivo studies exacerbate this contradiction because three separate strains of lgp2-dificient mice display different phenotypes.
Recently, a research team led by Prof. ZHANG Yibing from the Institute of Hydrobiology (IHB) of the Chinese Academy of Sciences provided more comprehensive evidence in vivo and in vitro that LGP2-knockout zebrafish is more susceptible to spring viraemia of carp virus (SVCV) infection than wild type zebrafish, and LGP2 deficiency severely disrupts the homeostatic regulation of host immune response to viral infection. The study was published in iScience.
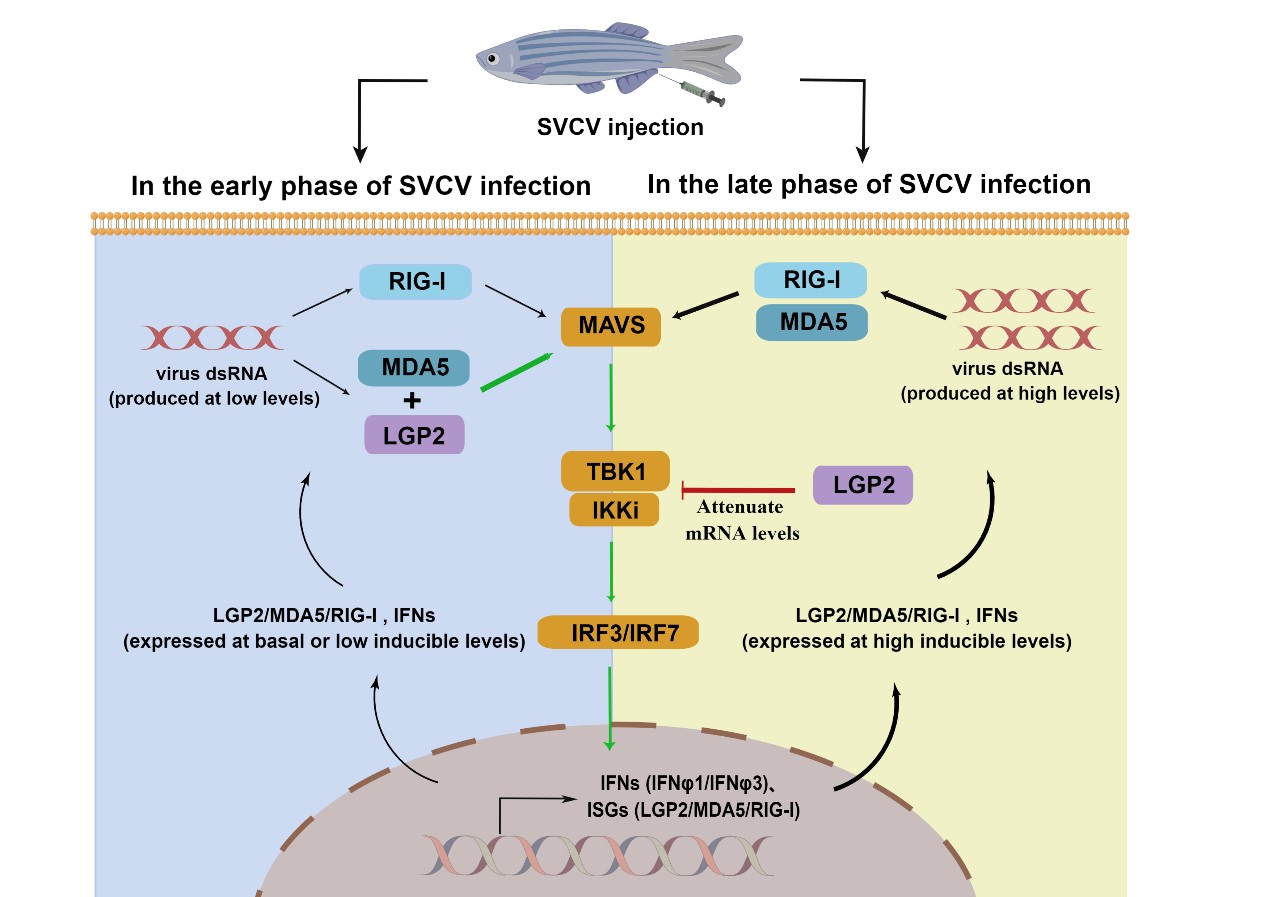
In this study, the researchers compared the immune response of wild type and LGP2 knockout zebrafish after virus infection, and found that lgp2-knockout zebrafish had insufficient IFN response in the early stage of virus infection and excessive IFN response in the late stage of viral infection. Mechanistically, LGP2 deficiency severely disrupts the homeostatic regulation of host immune response to viral infection.
At the same time, the researchers compared the immune response of cells when overexpression of LGP2 in vitro. They revealed that at the early stage of viral infection, zebrafish LGP2 functions as an essential activator of IFN response dependent on MDA5, but at the late stage, it functions as a negative regulator by impairing mRNA levels of tbk1 and ikki. The function switch of zebrafish LGP2 is dependent on a threshold level of IFN expression during viral infection.
In summary, this study revealed the key role of vertebrate LGP2s in antiviral immune homeostasis, and the biological significance of immune homeostasis in host defense against virus infection.
Zebrafish LGP2 maintains immune homeostasis to enable fish survival during virus infection (Credit: IHB)
(Editor: MA Yun)