Highlights
Manipulation of Cell Suspensions from Zebrafish Intestinal Mucosa Contributes to Understanding Enteritis
In aquaculture, feed-induced chronic inflammation of the intestinal mucosa is particularly common, and the intestinal mucosal immune barrier is often the first place where aquatic pathogens, such as viruses and bacteria, invade. Therefore, the study of fish enteritis has recently become a hotspot in basic aquatic disease research.
A complete set of enteritis research systems using zebrafish have previously been developed by researchers from the Institute of Hydrobiology (IHB) of the Chinese Academy of Sciences, including imaging of the intestinal mucosal immune cells and multi-omic analysis of soybean meal-induced enteritis (SBMIE). The zebrafish model of foodborne enteritis has been used in a series of aquatic drug development projects. However, the study of intestinal mucosal immunity at the cellular level requires a methodological breakthrough for related in-depth analysis.
Recently, the research group led by Prof. XIA Xiao-Qin from the Institute of Hydrobiology (IHB) of the Chinese Academy of Sciences innovated the preparation of immune cell suspensions from the zebrafish intestinal mucosa and developed a fast and simple operation method. The study was published in Frontiers in Immunology.
In this study, the researchers separated the immune cells above the villous muscular layer of zebrafish intestine by repeated pipetting. Compared to mesh rubbing method commonly used in farmed fish, the blowing method, which was by gently pipetting, resulted in less loss of viability of zebrafish mucosal tissue cells.
Using flow cytometry in combination with the gating strategy of fluorescently labeled immune cells, the researchers can accurately separate various immune cell groups in the zebrafish intestinal mucosal immune cell population, including neutrophils, macrophages, and lymphocytes.
Zebrafish strains labeled with immunocytofluorescence were used for isolation, and it was found that compared with the traditional mesh rubbing method, the cell viability detected by flow cytometry was greatly improved, the cells were basically free of adhesion, and the fluorescence expression and cell morphology were better.
Further, the transcriptomic results showed that the expression of immune cell marker genes was significantly higher in the isolated cell group than in the intestinal tissue itself, and the mucin gene expression heat map showed that the inner mucin gene was predominantly expressed in the isolated cell group. Enrichment analysis of DEGs (differential expression genes) also revealed a large number of immune-related pathways in the isolated cell group.
When analyzing the various types of immune cells (fluorescent markers) by flow cytometry, the researchers found that, compared with the mesh rubbing method, the blowing method can significantly increase the yield of immune cells in the cell suspension derived from intestinal mucosa. The application of the blowing method in the zebrafish SBMIE enteritis model demonstrated that this method is capable of accurately reflecting the changes in the proportion of enteritis-related immune cells, such as neutrophils and macrophages.
The blowing method can be applied to the pathological research of the mucosal barrier dysfunction caused by antigens and anti-nutritional factors, and even the functional ingredients added to the diet that are absorbed through the intestine.
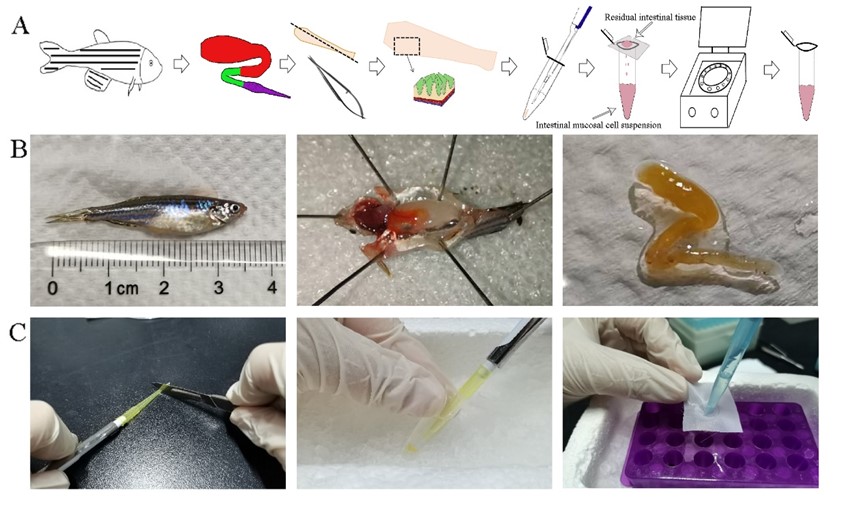
The schematic diagram for preparing zebrafish mucosal immune cell suspension (Image by IHB)
(Editor: MA Yun)