Highlights
Study Reveals a Novel Biological Axis Promotes Extraembryonic Development in Fish
During early embryonic development in fish, a transient extraembryonic structure known as the yolk syncytial layer (YSL) exists from the cleavage to the larval stage. Previous research has established that the YSL plays a critical role in inducing mesendoderm formation and driving epiboly movement. However, how the developmental fate of YSL, is regulated, and whether it possesses a zygotic genome activation (ZGA) program independent of the embryonic tissues, remain unclear.
Recent zebrafish research has identified the maternal factor Nanog as a critical regulator of early embryonic development and ZGA. The Giraldez team showed Nanog partners with pioneer factors Pou5f3 and Sox19b to initiate ZGA (Lee et al., Nature 2013; Miao et al., Mol Cell 2022), while the Zon team reported it activates the mxtx2-Nodal axis to promote mesendoderm formation (Xu et al., Dev Cell 2012). However, the Schier team proposed that Nanog's primary function is in extraembryonic tissue development (Gagnon et al., Development 2018). Nevertheless, the molecular mechanisms underlying Nanog regulation of extraembryonic tissues development and how Nanog regulation of embryonic and extraembryonic tissues might be orchestrated remain poorly understood.
Recently, in a study published in Science Bulletin, the research team led by Prof. SUN Yonghua from the Institute of Hydrobiology (IHB) of the Chinese Academy of Sciences reveals a "Nanog–cyp11a1–Pregnenolone (P5) axis" during oogenesis and early extraembryonic tissue development. This regulatory axis provides a comprehensive explanation for how Nanog promotes early embryonic extraembryonic tissue development and coordinates it with embryonic development.
In previous studies, this team generated Nanog loss-of-function mutants (He et al., Mutation Res, 2014) and made several key discoveries. They found that during oogenesis, Nanog acts as a transcriptional repressor that inhibits the translation elongation factor eef1a1l2, thereby exerting global translational control to promote oogenesis and early embryonic development (He & Jiao et al., Development 2022). Subsequently, they demonstrated that during early embryonic development, maternal Nanog binds to TCF factors to suppress the global activation of maternal β-catenin signaling, which is essential for the proper formation of the dorsoventral axis (He et al., PLOS Biology 2020).
In this latest publication, the research team revealed that the transcription of cyp11a1, which encodes cholesterol side-chain cleavage enzyme, was sharply down-regulated in the oocytes and early embryos of nanog mutants. Intriguingly, simple overexpression of cyp11a1 was sufficient to effectively rescue the epiboly defects, lethal developmental abnormalities, and aberrant cholesterol accumulation in the mutant embryos.
Further investigation identified a conserved Nanog–cyp11a1–P5 axis in oogenesis and early extraembryonic tissues. Specifically, Nanog activation of cyp11a1 during oogenesis drives cholesterol-to-P5 conversion, which is essential for microtubule assembly and oocyte maturation. These oogenic events, in turn, support microtubule assembly in the yolk cytoplasmic layer (YCL) of the early fertilized egg and the transport of maternal materials toward the animal pole.
Using knock-in lines for the pioneer factors Nanog, Pou5f3, and Sox19b, the researchers revealed that Nanog-mediated activation of cyp11a1 and P5 synthesis in the YSL nuclei is critical for initiating a downstream developmental cascade. This pathway promotes extraembryonic microtubule assembly, facilitates epiboly movement, and enables the nuclear accumulation of pioneer factors in the YSL. This process effectively activates a Nanog-cyp11a1-P5 axis-dependent ZGA program, leading to functional YSL formation and subsequent mesendoderm induction.
Furthermore, a characteristic genetic compensation mechanism was observed in MZcyp11a1 mutants, where the paralogous gene cyp11a2 was up-regulated in mutant oocytes and the YSL. This compensatory expression perfectly substituted for cyp11a1 by catalyzing P5 synthesis, thereby sustaining normal extraembryonic development and preventing early developmental defects in MZcyp11a1.
In conclusion, this research demonstrates that Nanog orchestrates embryonic cell fate and extraembryonic tissue integrity through cholesterol metabolism, microtubule dynamics, and maternal factors transport pathway. It also reveals a ZGA program within the YSL that operates independently of embryonic tissues. Simultaneously, this study offers a new perspective on the mechanism by which cholesterol metabolism regulates oocyte quality in fish.
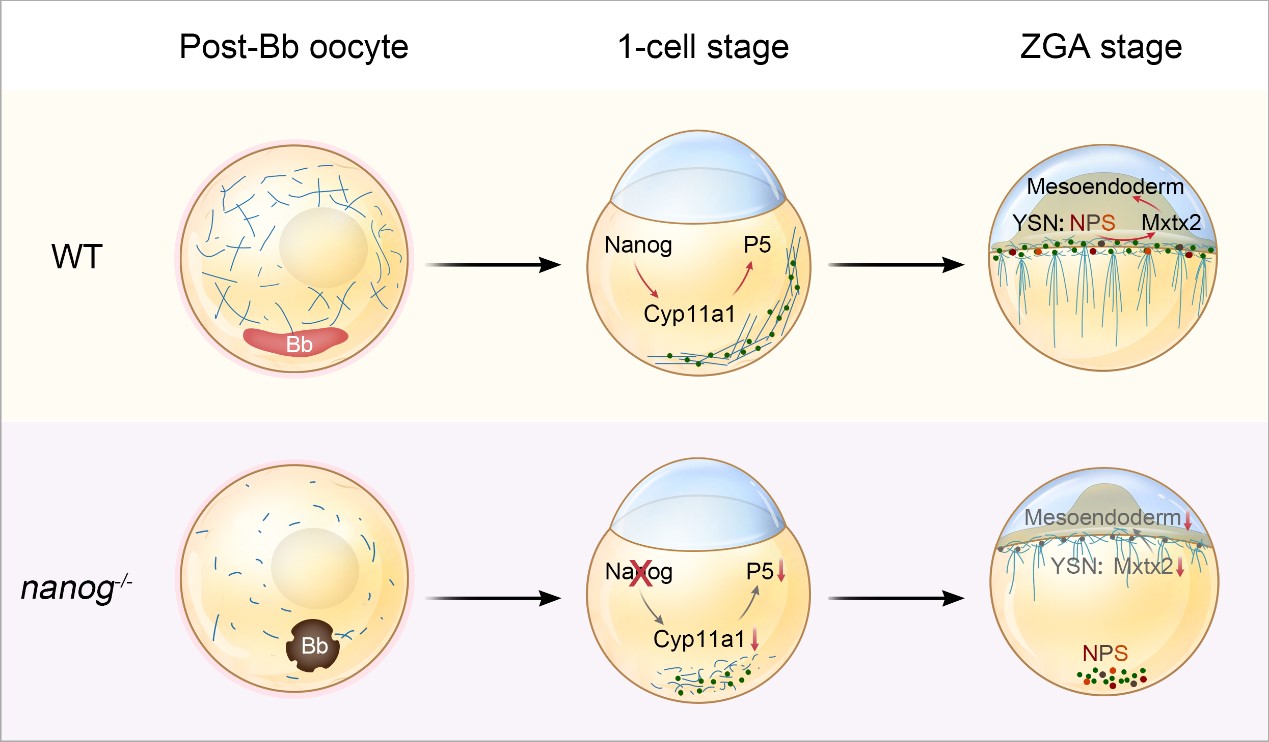
The molecular mechanism by which the Nanog-Cyp11a1-P5 axis guides extraembryonic tissue development. (Image by IHB)
(Editor: MA Yun)