Highlights
Study Reveals How Carbon Metabolism Regulates FtsZ Levels and Cell Division in Cyanobacteria
Bacteria surviving in fluctuating environments must tightly coordinate carbon metabolism and energy supply to sustain fundamental processes, growth, DNA replication, and division. However, the precise mechanisms underlying this complex regulatory network remain unclear. During bacterial division, the FtsZ protein acts as a pivotal molecular switch for cell division. By forming Z-rings and guiding septum assembly, it drives the contraction of the cell septum. Thus, elucidating the signaling mechanisms regulating FtsZ dynamics is key to understanding how carbon metabolism coordinates cell division.
As the sole prokaryotes capable of oxygenic photosynthesis, cyanobacteria exhibit distinctive carbon metabolism pathways. They rely on the Calvin-Benson cycle for carbon fixation, and their tricarboxylic acid (TCA) cycle remains in an ‘open’ state due to the absence of α-ketoglutarate dehydrogenase, resulting in metabolic flux patterns markedly different from those of heterotrophic bacteria. Although prior studies suggest that nutrient conditions influence cyanobacterial division, the underlying regulatory pathways remain elusive.
Recently, in a study published in Communications Biology, a research group led by Professor ZHANG Cheng-Cai from the Institute of Hydrobiology (lHB) of the Chinese Academy of Sciences establishes a model in which metabolic flux reprogramming regulates cell division in cyanobacteria. This study offers new insights into the coupling mechanism between cell division and carbon metabolism in prokaryotes.
In this study, Zhang's team introduced an artificial CO₂ fixation module (PCEM) consisting of crotonyl-CoA carboxylase/reductase (Ccr) into the TCA pathway of the filamentous cyanobacterium Anabaena sp. PCC 7120. The engineered strain gPCEM exhibited light-dependent division, being blocked under low light and accelerated under high light.
Further studies showed that high light intensity drove enhanced carbon metabolic flow and contributed to the rise in the content of the metabolite α-ketoglutarate (2-OG), which acts as a signaling molecule to enhance the binding of the global transcription factor NtcA to the ftsZ promoter, thereby up-regulating the expression of FtsZ, which in turn affected the cell division process.
This study is the first to outline the regulatory pathway of "carbon metabolism-NtcA (C/N balance-responsive transcription factor)-FtsZ-cell division" in cyanobacteria, and systematically elucidated the mechanism of coordinated carbon metabolism in regulating cell division. This work systematically elucidates how carbon metabolism coordinately regulates cell division, providing a new paradigm for understanding the synergistic mechanism of metabolism and division in photosynthetic organisms, and a theoretical basis for synthetic biology.
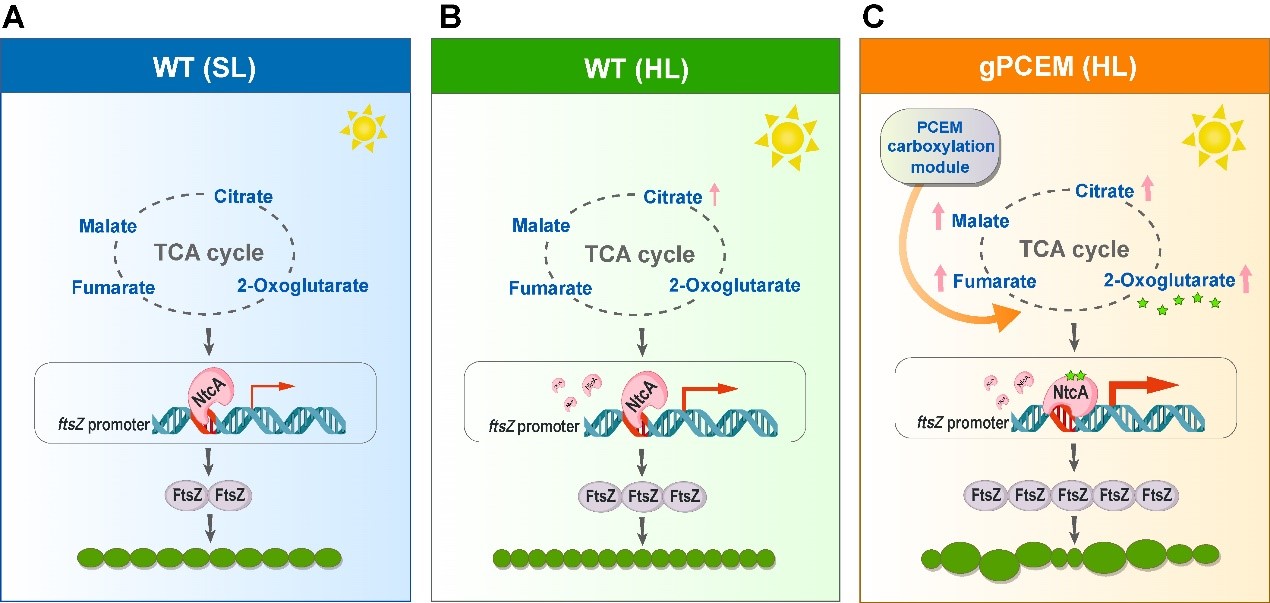
A model for NtcA-regulated FtsZ levels and cell division in Anabaena (Image by IHB)
(Editor: MA Yun)