
Newsroom
Researchers Reveal Functional Divergence of Multiple Duplicated Foxl2 Homeologs and Alleles in A Recurrent Polyploid Fish
In a study published in Molecular Biology and Evolution, a research group led by Prof. GUI Jianfang from the Institute of Hydrobiology (IHB) of the Chinese Academy of Sciences elaborated the functional divergence of multiple duplicated foxl2 - a central player in ovary - homeologs and alleles in gibel carp (Carassius gibelio), a recurrent auto-allo-hexaploid fish.
Polyploidy is considered as a major driver of speciation and shapes almost every aspect of species evolution. The neopolyploid might firstly undergo a chaos of “genomic shock”, then occur a series of complicated non-Mendelian changes to shape a novel genome, and finally evolve into a paleopolyploid or a new diploid.
The duplicated genes may evolve into homeolog or allele after allopolyploidy or autopolyploidy event respectively. In previous study, IHB researchers have found that zebrafish has two foxl2 paralogs (foxl2a and foxl2b), which sequentially and cooperatively regulate ovary development and maintenance (Yang et al., Genetics, 2017, 205:1551-1572.). Although the fates of duplicated genes have been well documented in paleopolyploids, the research is scarce in recurrent polyploids with complex genomes.
The researchers first identified three divergent foxl2 homeologs (Cgfoxl2a-B, Cgfoxl2b-A, and Cgfoxl2b-B) and found that each of them possessed three highly conserved alleles in gibel carp and Cgfoxl2a-A gene might have been lost in the ancestor of Carassius complex after allotetraploidy event.
They then uncovered the abundant sexual dimorphism and biased expression of these multiple duplicated foxl2 genes in hypothalamic-pituitary-gonadal axis. The interactions among granulosa cells, theca cells and oocytes are pivotal to folliculogenesis, steroidogenesis and female oogenesis.
By distinct cellular localization of CgFoxl2a and CgFoxl2b, the researchers distinguished granulosa cells and three subpopulations of thecal cells, and traced their functional roles and the involved process in folliculogenesis.
Finally, they successfully edited multiple foxl2 homeologs and/or alleles in gibel carp by using CRISPR/Cas9, and found that Cgfoxl2a-B deficiency led to ovary development arrest or complete sex reversal in a dosage-dependent manner, while the mutated Cgfoxl2a-B allele with an intact Forkhead domain still can activate Cgcyp19a1a-A expression.
When the researchers completely disrupted the expression of Cgfoxl2b-A and Cgfoxl2b-B, they found, to their surprise, that the gonad of Cgfoxl2b-A and Cgfoxl2b-B double-mutated individuals lost germ cell and the compensatory increase of Cgfoxl2a-Bs transcripts could not rescue the defect of germ cell depletion.
Moreover, compared to Cgfoxl2b-A and Cgfoxl2b-B double mutated individuals (15.3% with ovotestis and testis), they observed more F0 individuals with mutated Cgfoxl2a-B, Cgfoxl2b-A and Cgfoxl2b-B (28.9%) occurred partial or complete ovary to testis sex reversal.
Taken together, the detailed cellular localization and functional differences indicate that Cgfoxl2a and Cgfoxl2b have subfunctionalized and cooperated to regulate folliculogenesis and gonad differentiation, and Cgfoxl2b has evolved a new function in oogenesis.
This study covers almost all ranges of complex evolutionary scenarios associated with duplicated genes, including homeolog/allele diversification, retention/loss, biased expression, and sub-/neofunctionalization. Although theoretical presumption of these scenarios and their disparate cases have already been reported, their work has elaborated the evolutionary mechanisms involved in the duplicated foxl2 gene.
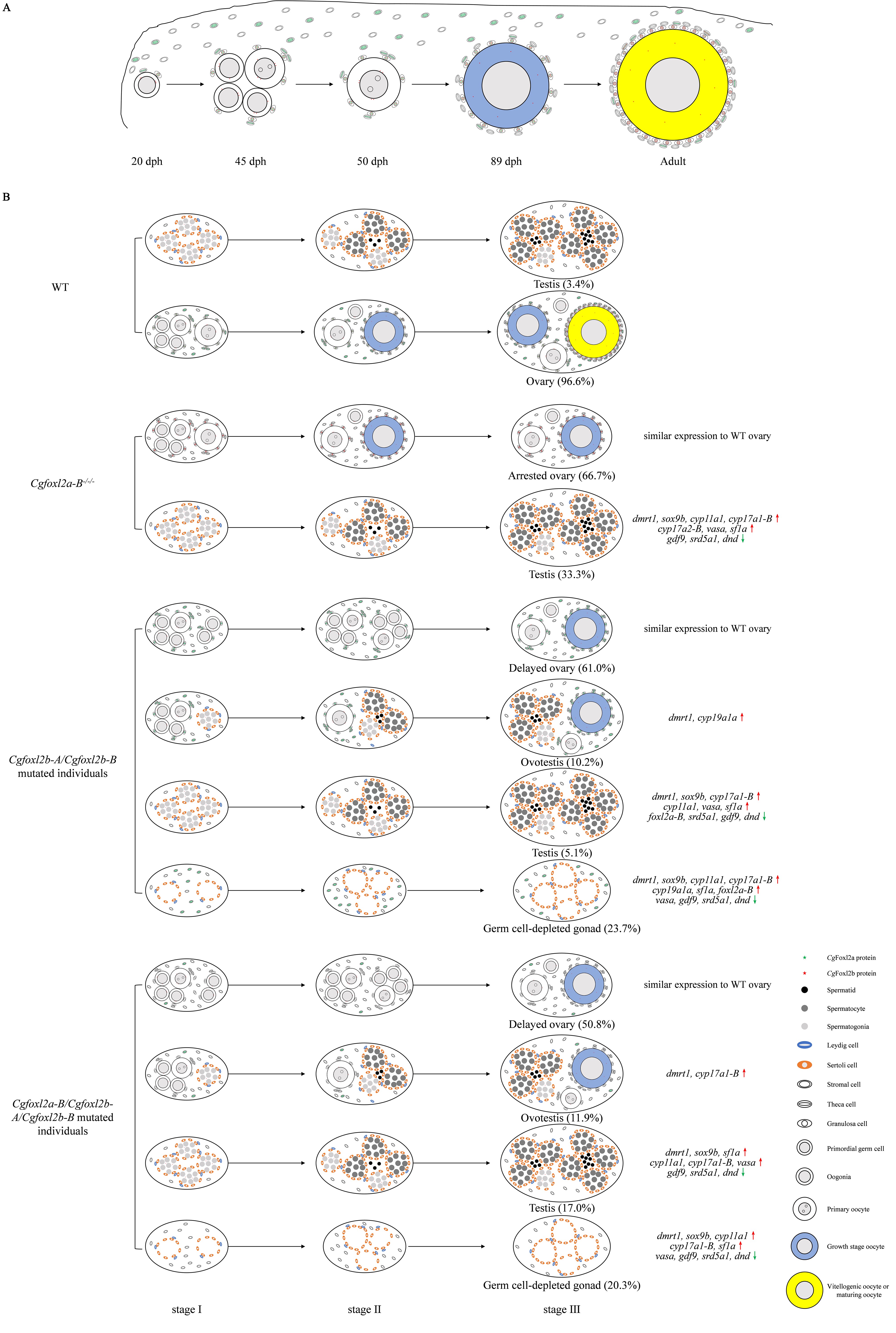
Schematic diagram of folliculogenesis (A) and cooperative regulation between Cgfoxl2a and Cgfoxl2b (B) in gibel carp ovary development. (Credit: IHB)