
Newsroom
Redox Signal Regulates Multiciliary Coordinated Beating
In a study published in PNAS, two research groups led by Prof. HUANG Kaiyao from the Institute of Hydrobiology (IHB) of the Chinese Academy of Sciences and Prof. ZHAO Chengtian from Ocean University of China revealed the important role ofcytochrome b5-like/heme-binding domain1 (CYB5D1) protein in the coordinated beating of multiple cilia.
Coordinated beating of multiple cilia on epithelial surfaces, for example, in the trachea, oviduct, and brain ventricles of vertebrates, is crucial for mucus clearance in the airway, ovum transport in the oviduct, and cerebrospinal fluid circulation in the brain ventricles. Impairment of coordinated beating of these multiple cilia in humans can result in ciliopathies such as chronic respiratory problems, infertility, and hydrocephalus. However, the molecular mechanism is poorly understood.
CYB5D1 contains a heme-binding domain and a cordon-bleu ubiquitin-like domain and is highly conserved in the motile ciliated organisms. Researcher knocked out Cyb5d1 in Zebrafish using CRISP/Cas9 and found the number of otoliths in otic vesicle increased (Fig. 1 A), which is similar to the phenotype of radial spoke proteins mutants.
The researchers then obtained the CYB5D1 mutant in Chlamydomonas reinhardtii (Crcyb5d1) by forward genetics method. Results showed that the swimming velocity was dramatically reduced, the cells were rocking in place and the tracks were short and kinked in Crcybd51 (Fig. 1 B).
Moreover, the beating waveform of flagella in Wild-type and Crcyb5d1 was observed and recorded in real-time using high-speed video microscopy. The two flagella of most Wild-type cells beat coordinately, the power stroke or recovery stroke of the two flagella coincided synchronously, resembling breaststroke swimming (Fig. 1 C).
In contrast, the coordinated flagellar beating is impaired in Crcyb5d1, when the two flagella finished the recovery stroke. The trans-flagellum began a power stroke, but the cis-flagellum was drawn back to the cell body immediately with a curled shape (Fig. 1 C).
The area encompassing the beat envelope of the cis- and trans-flagella is almost the same in Wild-type, while it is dramatically decreased in the cis-flagellum of Crcyb5d1 (Fig.1 D). These results suggested a defect in the cis-flagellum of Crcyb5d1.
Similarly, the coordinated ciliary beating in the otic vesicle and olfactory epithelium is also severely impaired in Cyb5d1 zebrafish mutant, suggesting that the function of CYB5D1 in the coordinated ciliary beating is conserved in the motile ciliated organisms.
To understand how CYB5D1 controls the flagellar coordinated beating in the molecular mechanism level, the researchers performed biochemical and genetic analyses and revealed that CYB5D1 is a novel radial spoke stalk protein (Fig. 2 A and B). CYB5D1 binds heme through its heme-binding domain in the N terminus, and the binding activity is redox-sensitive, only oxidized CYB5D1 binds heme.
Lack of CYB5D1 resulted in a reductive shift in flagellar redox state. Treatment of Crcyb5d1 with oxidants restored coordinated flagellar beating (Fig. 2 C and D).
Taken together, the data suggest that redox is a novel signal regulating the coordinated ciliary beating. CYB5D1 may integrate environmental and intra-ciliary signals, and regulate the redox state of cilia, which is crucial for the coordinated beating of multiple cilia (Fig. 2 E). The response to redox signal of two flagella is different in Chlamydomonas, which is the basis for the cell body to change the swimming direction.
The findings also revealed the important role of redox in the coordinated beating of multiple cilia and suggested a possible ciliopathy therapy by modulating the redox poise in cilia.
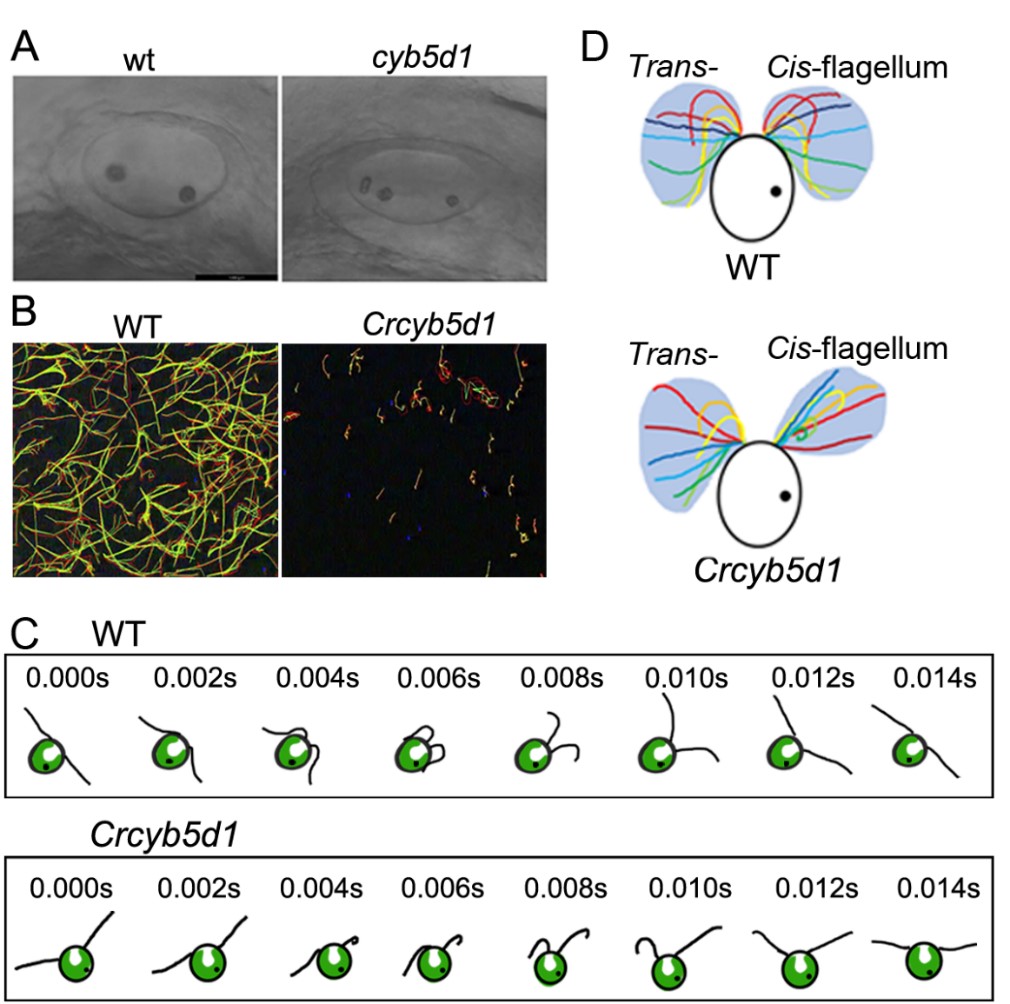
Figure 1. CYB5D1 is essential for ciliary coordinated beating in Zebrafish and Chlamydomonas. (A) The number of otoliths in the otic vesicles is increased in cyb5d1 mutant at 24 hpf embryos. (B) The track of Wild-type is long and smooth, while the track of Crcybd51 is short and kinked. (C) Schematic diagram of two flagella beating waveforms in Wild-type and Crcyb5d1 in real-time under high-speed video microscopy. The two flagella of most Wild-type cells beat coordinated, the power stroke or recovery stroke of the two flagella coincided synchronously. In contrast, the coordinated flagellar beating is impaired in Crcyb5d1, when the two flagella finished the recovery stroke, the trans-flagellum began a power stroke, but the cis-flagellum was drawn back to the cell body immediately with a curled shape. (D) The area encompassing the beat envelope of the trans-flagellum is about double that of the cis-flagellum in Crcyb5d1, while they are similar in Wild-type. (Image by IHB)
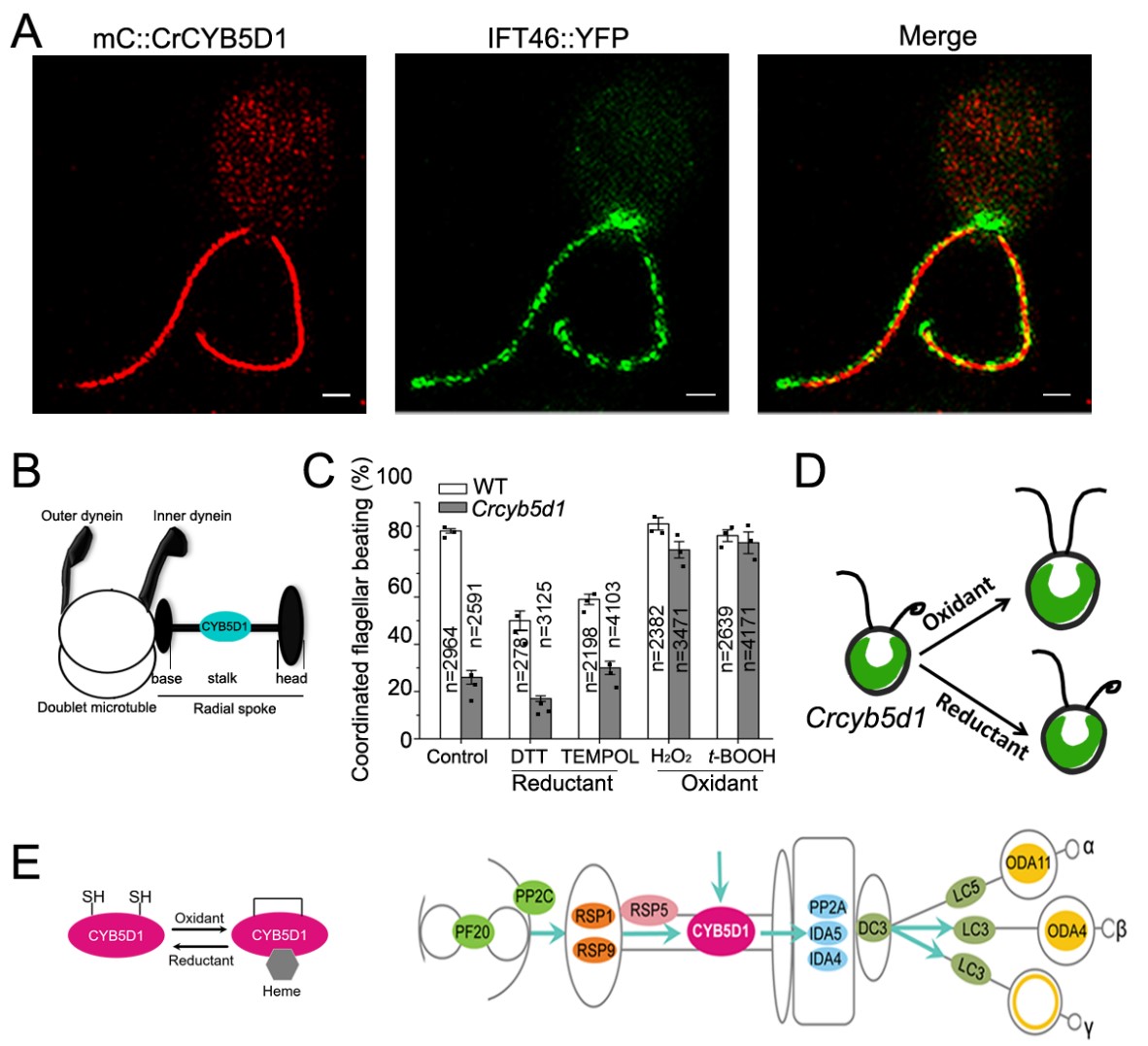
Figure 2. CYB5D1 is a novel radial spoke component and regulates coordinated ciliary beating by redox. (A) The mC::CrCYB5D1 exhibited a strong and continuous signal in the flagella, which was surrounded by the signal from co-expressed IFT46::YFP. IFT46::YFP is a marker of the flagellar matrix and present in intraflagellar transport trains. (B) Schematic diagram shows CYB5D1 localizes to the radial spoke stalk. Inner dynein, Outer dynein, and Radial spoke are flagellar motility-associated complexes. Each radial spoke consists of a base, stalk and head. (C) Percentage of coordinated flagellar beating in Wild-type is decreased after reductants treatment, and it is increased in Crcyb5d1 following treatment with oxidants. (D) Schematic diagram shows reductants do not affect the coordinated flagellar beating, while the oxidants restored the uncoordinated flagellar beating of Crcyb5d1. (E) CYB5D1 binds heme in redox-sensitive, only oxidized CYB5D1 binds heme. CYB5D1 may integrate environmental and intra-ciliary redox signal, and transmit it to inner dynein and outer dynein through radial spoke. All the indicated proteins in the flagellar motility-associated complexes are redox-sensitive. (Image by IHB)